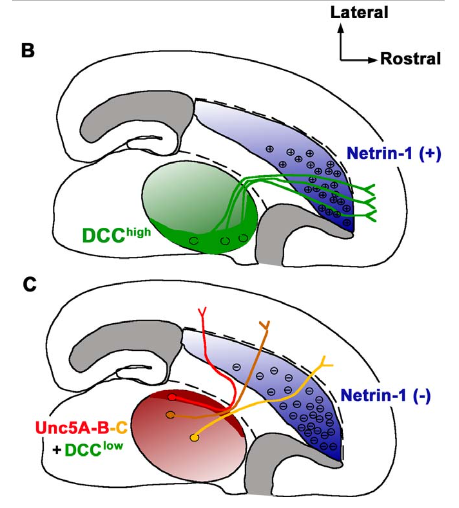
The functional properties of each structure in the central nervous system are critically dependent on the precision of neuronal connectivity. The cerebral cortex in particular is a highly organized structure divided into many distinct cortical areas underlying important sensory, motor, and cognitive functions in the brain. Each primary cortical area receives its synaptic inputs from the periphery via the dorsal thalamus. The main relay station for sensory information to the cortex, the thalamus, can be divided into specific nuclei projecting topographically to individual cortical areas. How is the complex topography of thalamic axon projection to individual cortical areas specified during development? Recent evidence demonstrated that thalamic axons are routed to different cortical domains before they enter the cortex, by putative axon guidance cues present in the ventral forebrain. In the present study, we provide evidence that a secreted axon guidance cue, Netrin-1, expressed in a long-range gradient in the ventral forebrain, plays a critical role in the establishment of the topography of thalamic projections by directing different subsets of axons to specific cortical domains. These results provide important insights into the molecular mechanisms responsible for shaping the topographical patterns of thalamocortical axon projections in mammals.