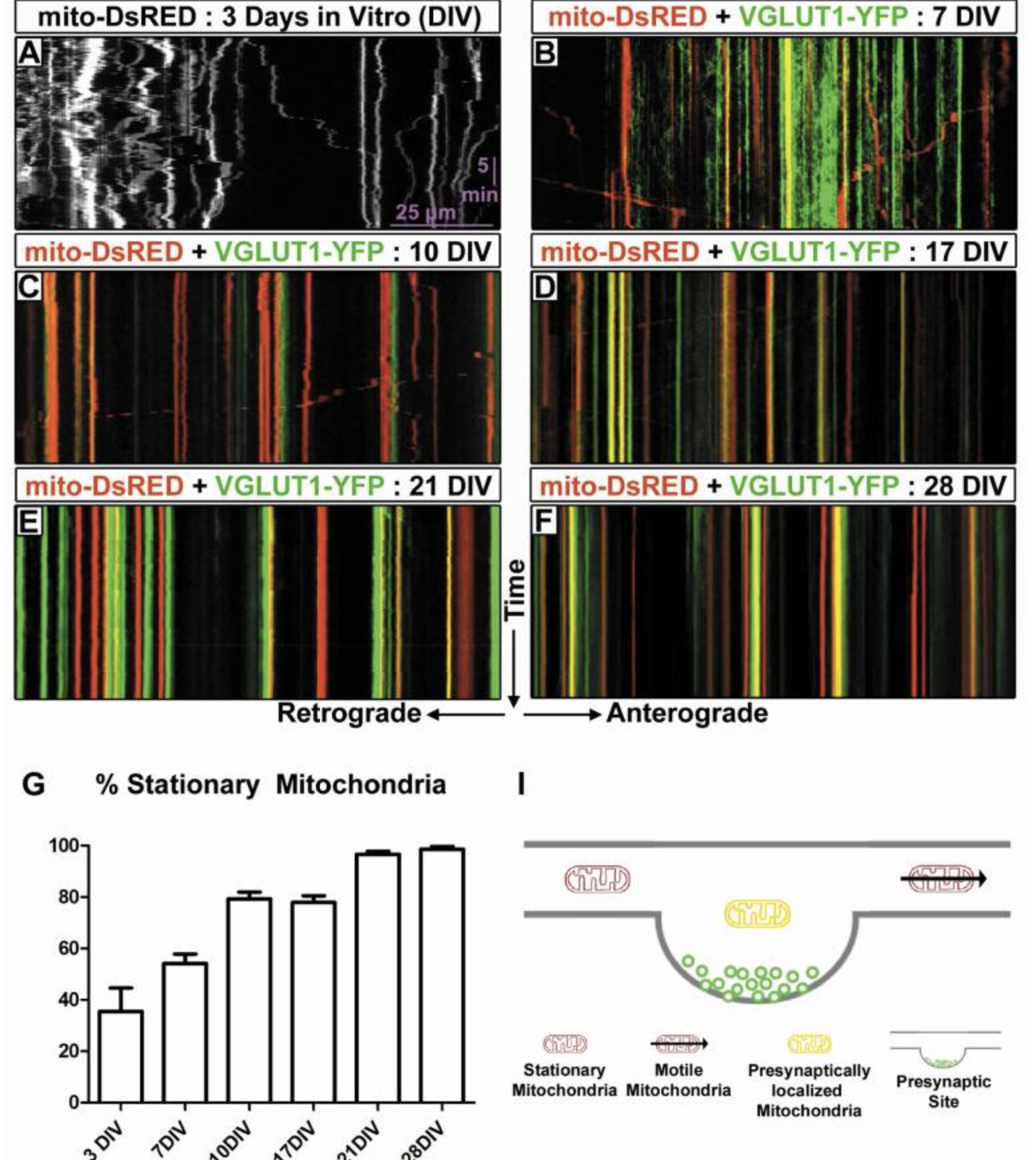
The importance of mitochondria for neuronal function is evident by the large number of neurodegenerative diseases that have been associated with a disruption of mitochondrial function or transport (reviewed in [1, 2]). Mitochondria are essential for proper biological function as a result of their ability to produce ATP through oxidative phosphorylation, buffer cytoplasmic calcium, regulate lipid biosynthesis, and trigger apoptosis (reviewed in [2]). Efficient transport of mitochondria is thought to be particularly important in neurons in light of their compartmentalization, length of axonal processes, and high-energy requirements (reviewed in [3]). However, the majority of these results were obtained using short-term, in vitro neuronal culture models, and very little is currently known about mitochondrial dynamics in mature axons of the mammalian CNS in vitro or in vivo. Furthermore, recent evidence has demonstrated that mitochondrial immobilization at specific points along the axon, such as presynaptic boutons, play critical roles in axon morphogenesis [4, 5]. We report that as cortical axons mature, motility of mitochondria (but not other cargoes) is dramatically reduced and this coincides with increased localization to presynaptic sites. We also demonstrate using photo-conversion that in vitro mature axons display surprisingly limited long-range mitochondrial transport. Finally, using in vivo two-photon microscopy in anesthetized or awake-behaving mice, we document for the first time that mitochondrial motility is also remarkably low in distal cortical axons in vivo. These results argue that mitochondrial immobilization and presynaptic localization are important hallmarks of mature CNS axons both in vitro and in vivo.