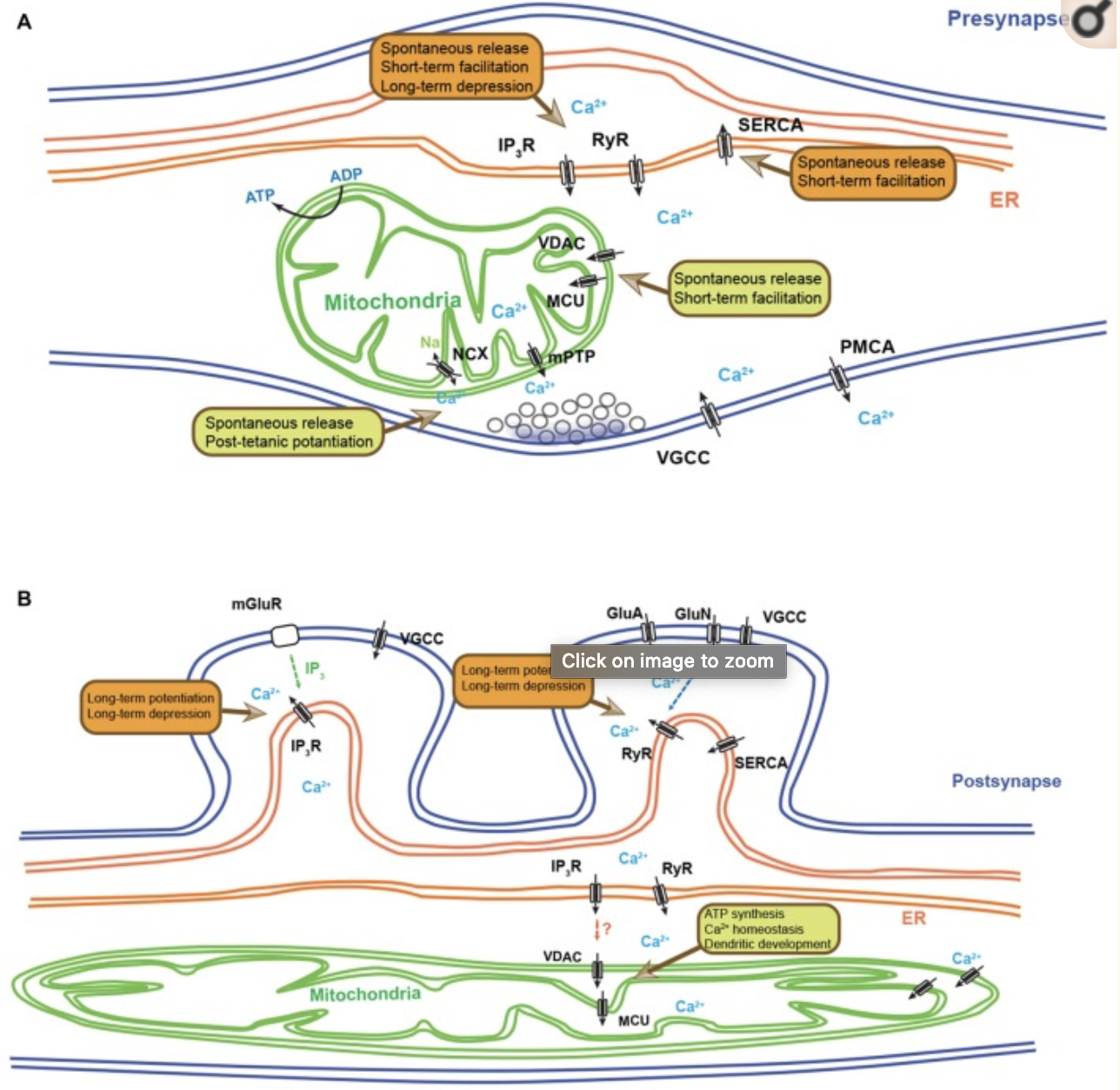
Calcium (Ca2+) plays innumerable critical functions in neurons ranging from regulation of neurotransmitter release and synaptic plasticity to activity-dependent transcription. Therefore, more than any other cell types, neurons are critically dependent on spatially and temporally controlled Ca2+ dynamics. This is achieved through an exquisite level of compartmentalization of Ca2+ storage and release from various organelles. The function of these organelles in the regulation of Ca2+ dynamics has been studied for decades using electrophysiological and optical methods combined with pharmacological and genetic alterations. Mitochondria and the endoplasmic reticulum (ER) are among the organelles playing the most critical roles in Ca2+ dynamics in neurons. At presynaptic boutons, Ca2+ triggers neurotransmitter release and synaptic plasticity, and postsynaptically, Ca2+ mobilization mediates long-term synaptic plasticity. To explore Ca2+ dynamics in live cells and intact animals, various synthetic and genetically encoded fluorescent Ca2+ sensors were developed, and recently, many groups actively increased the sensitivity and diversity of genetically encoded Ca2+ indicators (GECIs). Following conjugation with various signal peptides, these improved GECIs can be targeted to specific subcellular compartments, allowing monitoring of organelle-specific Ca2+ dynamics. Here, we review recent findings unraveling novel roles for mitochondria- and ER-dependent Ca2+ dynamics in neurons and at synapses.