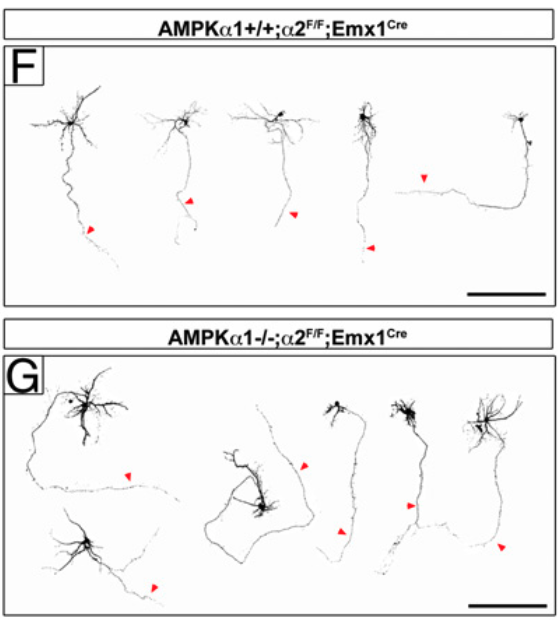
Mammalian brain connectivity requires the coordinated production and migration of billions of neurons and the formation of axons and dendrites. The LKB1/Par4 kinase is required for axon formation during cortical development in vivo partially through its ability to activate SAD-A/B kinases. LKB1 is a master kinase phosphorylating and activating at least 11 other serine/threonine kinases including the metabolic sensor AMP-activated protein kinase (AMPK), which defines this branch of the kinome. A recent study using a gene-trap allele of the β1 regulatory subunit of AMPK suggested that AMPK catalytic activity is required for proper brain development includ- ing neurogenesis and neuronal survival. We used a genetic loss- of-function approach producing AMPKα1/α2-null cortical neurons to demonstrate that AMPK catalytic activity is not required for cortical neurogenesis, neuronal migration, polarization, or survival. However, we found that application of metformin or AICAR, potent AMPK acti- vators, inhibit axogenesis and axon growth in an AMPK-dependent manner. We show that inhibition of axon growth mediated by AMPK overactivation requires TSC1/2-mediated inhibition of the mamma- lian target of rapamycin (mTOR) signaling pathway. Our results dem- onstrate that AMPK catalytic activity is not required for early neural development in vivo but its overactivation during metabolic stress impairs neuronal polarization in a mTOR-dependent manner.