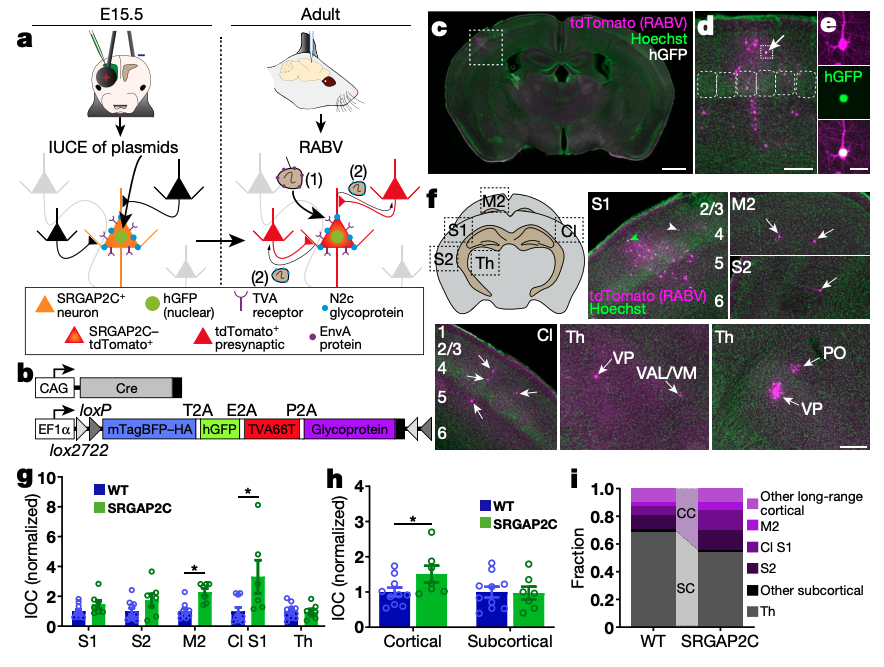
The cognitive abilities that characterize humans are thought to emerge from unique features of the cortical circuit architecture of the human brain, which include increased cortico-cortical connectivity. However, the evolutionary origin of these changes in connectivity and how they affected cortical circuit function and behaviour are currently unknown. The human-specific gene duplication SRGAP2C emerged in the ancestral genome of the Homo lineage before the major phase of increase in brain size1,2. SRGAP2C expression in mice increases the density of excitatory and inhibitory synapses received by layer 2/3 pyramidal neurons (PNs)3-5. Here we show that the increased number of excitatory synapses received by layer 2/3 PNs induced by SRGAP2C expression originates from a specific increase in local and long-range cortico-cortical connections. Mice humanized for SRGAP2C expression in all cortical PNs displayed a shift in the fraction of layer 2/3 PNs activated by sensory stimulation and an enhanced ability to learn a cortex-dependent sensory-discrimination task. Computational modelling revealed that the increased layer 4 to layer 2/3 connectivity induced by SRGAP2C expression explains some of the key changes in sensory coding properties. These results suggest that the emergence of SRGAP2C at the birth of the Homo lineage contributed to the evolution of specific structural and functional features of cortical circuits in the human cortex.